A 246/2013. (VII. 2.) Gov. decree
rendelet a kozmetikai termékekről az Európai Parlament 1223/2009/EK rendeletével együtt ad szabályokat a kozmetikai termékekre Magyarországon.
A rendelet külön foglakozik a kozmetikai termékek címkézésével, megerősíti az EK rendeletében előírtakat azzal a kikötéssel, hogy a rendeletben említett tájékoztatón bizonyos információkat magyar nyelven kell feltüntetni.
The label should show in Hungarian:
-
1. b) the nominal content at the time of packaging, given by
weight or by volume, except in the case of packaging containing
less than five grams or five millilitres, free samples
and single-application packs; for pre-packages normally sold
as a number of items, for which details of weight or volume
are not significant, the content need not be given provided
the number of items appears on the packaging. This information
need not be given if the number of items is easy to
see from the outside or if the product is normally only sold
individually;
-
1. c) the date until which the cosmetic product, stored under
appropriate conditions, will continue to fulfil its initial function
and, in particular, will remain in conformity with
Article 3 (‘date of minimum durability’).
The date itself or details of where it appears on the packaging
shall be preceded by the symbol shown in point 3 of
Annex VII

or the words: ‘best used before the end of’.
The date of minimum durability shall be clearly expressed
and shall consist of either the month and year or the day,
month and year, in that order.
If necessary, this information
shall be supplemented by an indication of the conditions
which must be satisfied to guarantee the stated durability.
Indication of the date of minimum durability shall not be
mandatory for cosmetic products with a minimum durability
of more than 30 months. For such products, there shall
be an indication of the period of time after opening for which
the product is safe and can be used without any harm to the
consumer. This information shall be indicated, except where
the concept of durability after opening is not relevant, by the
symbol shown in point 2 of Annex VII
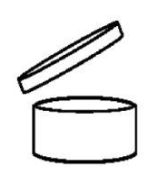
followed by the period
(in months and/or years);
- 1. d) particular precautions to be observed in use, and at least
those listed in Annexes III to VI and any special precautionary
information on cosmetic products for professional use;
- 1. f) the function of the cosmetic product, unless it is clear from
its presentation;
Where it is impossible for practical reasons to label the
information mentioned here, the following applies:
- the information shall be mentioned on an enclosed or
attached leaflet, label, tape, tag or card;
- unless impracticable, this information shall be referred to by
abbreviated information or the symbol given in point 1 of
Annex VII, which must appear on the container or packaging
for the information referred in point (d) of paragraph 1
and on packagging for the information referred in point (g) of
paragraph 1 of 1223/2009/EC.
In the case of soap, bath balls and other small products
where it is impossible for practical reasons for the information
referred to in point (g) of paragraph 1 to appear on a label, tag,
tape or card or in an enclosed leaflet, this information shall appear
on a notice in immediate proximity to the container in which the
cosmetic product is exposed for sale.
For cosmetic products that are not pre-packaged, are packaged
at the point of sale at the purchaser’s request, or are prepackaged
for immediate sale.
1 million HUF fine for non complete label
The manufacturer or distributor on label is suitable for lawful identification and customer information if it also shows distributor or manufacturer role - says the High Court.